Intro
Discover 5 BYU chemical tips for safe handling, storage, and disposal, covering lab safety protocols, hazardous materials, and emergency procedures.
The world of chemistry can be fascinating and intimidating at the same time, especially for those who are new to the field. With so many chemicals and reactions to learn about, it can be overwhelming to know where to start. However, with the right tips and guidance, anyone can become proficient in chemistry and unlock its many secrets. In this article, we will explore five BYU chemical tips that can help you improve your understanding of chemistry and become a master of the subject.
Chemistry is all around us, from the air we breathe to the food we eat. It is a fundamental part of our daily lives, and understanding its principles can help us make informed decisions about our health, our environment, and our future. Whether you are a student, a researcher, or simply someone who is curious about the world around you, chemistry has something to offer. With these five BYU chemical tips, you can take your knowledge of chemistry to the next level and unlock a world of possibilities.
From the basics of atomic structure to the complexities of organic synthesis, chemistry is a vast and fascinating field that requires patience, dedication, and practice to master. However, with the right approach and mindset, anyone can become proficient in chemistry and achieve their goals. Whether you are looking to improve your grades, advance your career, or simply satisfy your curiosity, these five BYU chemical tips can help you get started on your journey to chemical mastery.
Understanding Chemical Reactions
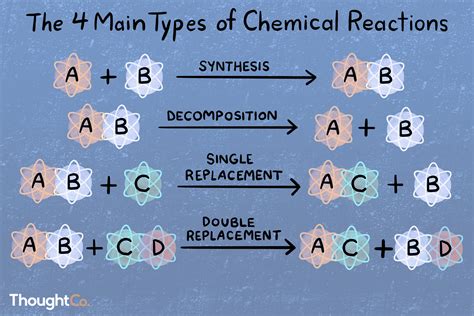
To understand chemical reactions, it is essential to have a solid grasp of chemical bonding and the principles of thermodynamics. Chemical bonding refers to the attractive and repulsive forces that hold atoms together, while thermodynamics is the study of energy and its relationship to chemical reactions. By understanding these principles, you can better appreciate the mechanisms of chemical reactions and predict their outcomes.
Key Principles of Chemical Reactions
Some key principles of chemical reactions include: * The law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction * The law of conservation of energy, which states that energy cannot be created or destroyed in a chemical reaction * The principle of equilibrium, which states that chemical reactions tend towards a state of balance and stability * The principle of kinetics, which studies the rates of chemical reactions and the factors that influence themMastering Chemical Equations
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. By understanding these relationships, you can balance chemical equations and predict the amounts of substances required or produced in a reaction. Some key principles of stoichiometry include:
- The mole concept, which is a unit of measurement for amounts of substances
- The concept of molar mass, which is the mass of a substance per mole
- The concept of limiting reactants, which determines the amount of product formed in a reaction
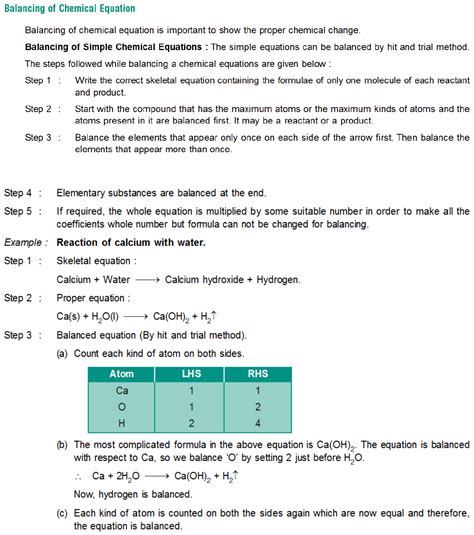
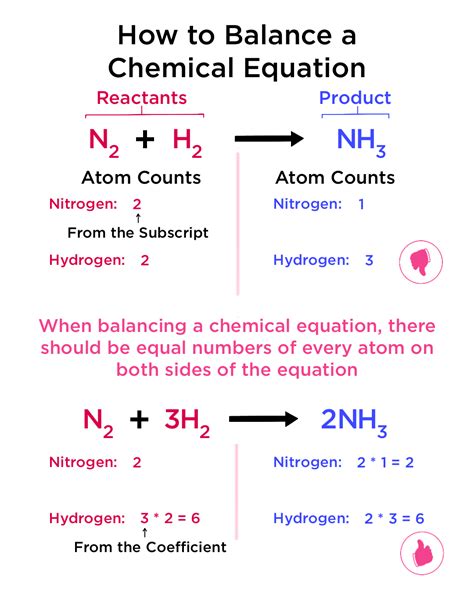
Steps to Balance Chemical Equations
To balance a chemical equation, follow these steps: 1. Write the unbalanced equation, using formulas and symbols to represent the reactants and products. 2. Count the number of atoms of each element on both sides of the equation. 3. Add coefficients to the formulas to balance the equation, starting with the elements that appear most frequently. 4. Check the equation to ensure that it is balanced and that the coefficients are whole numbers.Understanding Chemical Properties
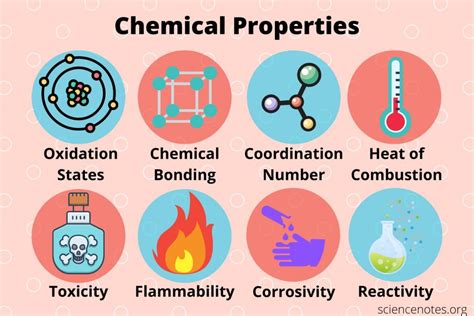
Types of Chemical Properties
There are many different types of chemical properties, including: * Intensive properties, which are independent of the amount of substance present * Extensive properties, which depend on the amount of substance present * Physical properties, which can be observed or measured without changing the substance's chemical composition * Chemical properties, which can only be observed or measured by changing the substance's chemical compositionUsing Laboratory Equipment
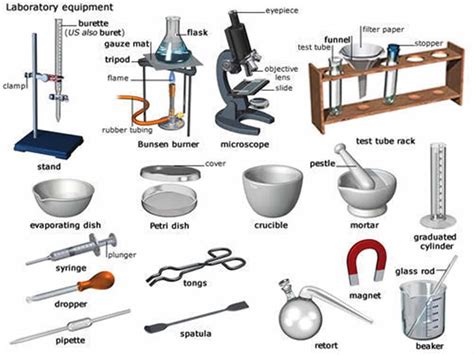
Some key principles of laboratory equipment include:
- Calibration, which ensures that the equipment is accurate and reliable
- Maintenance, which ensures that the equipment is in good working order
- Safety, which ensures that the equipment is used safely and responsibly
Steps to Use Laboratory Equipment
To use laboratory equipment, follow these steps: 1. Read the manual and understand the equipment's principles and operation. 2. Calibrate the equipment according to the manufacturer's instructions. 3. Use the equipment according to the manufacturer's instructions and proper safety protocols. 4. Maintain the equipment regularly to ensure that it is in good working order.Analyzing Chemical Data

Some key principles of chemical data analysis include:
- Statistical analysis, which is used to analyze and interpret the results of experiments and measurements
- Data visualization, which is used to represent and communicate the results of experiments and measurements
- Data interpretation, which is used to draw conclusions and make decisions based on the results of experiments and measurements
Steps to Analyze Chemical Data
To analyze chemical data, follow these steps: 1. Collect and organize the data according to the experiment or measurement. 2. Use statistical analysis to analyze and interpret the data. 3. Use data visualization to represent and communicate the results. 4. Draw conclusions and make decisions based on the results.BYU Chemical Image Gallery



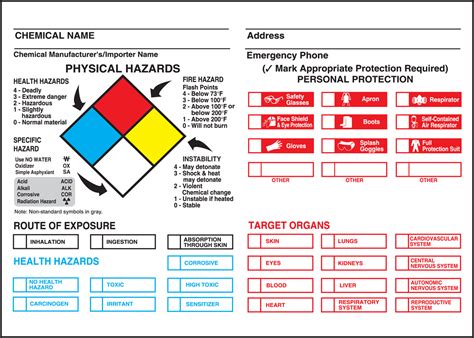

What are the key principles of chemical reactions?
+The key principles of chemical reactions include the law of conservation of mass, the law of conservation of energy, the principle of equilibrium, and the principle of kinetics.
How do I balance a chemical equation?
+To balance a chemical equation, write the unbalanced equation, count the number of atoms of each element on both sides, and add coefficients to the formulas to balance the equation.
What are the different types of chemical properties?
+The different types of chemical properties include physical properties, chemical properties, and spectroscopic properties.
How do I use laboratory equipment safely and effectively?
+To use laboratory equipment safely and effectively, read the manual, calibrate the equipment, use it according to the manufacturer's instructions, and maintain it regularly.
What are the key principles of chemical data analysis?
+The key principles of chemical data analysis include statistical analysis, data visualization, and data interpretation.
By following these five BYU chemical tips, you can improve your understanding of chemistry and become a master of the subject. Remember to always follow proper safety protocols and procedures when working with chemicals and laboratory equipment, and to use statistical analysis and data interpretation to analyze and interpret chemical data. With practice and dedication, you can unlock the secrets of chemistry and achieve your goals. Share your thoughts and experiences with chemistry in the comments below, and don't forget to share this article with your friends and colleagues who may be interested in learning more about chemistry.
