Intro
Unlock 5 essential chem tips, including lab safety, chemical reactions, and experiment techniques, to enhance your chemistry skills and knowledge with practical applications and expert advice.
Chemistry is a vital part of our daily lives, from the air we breathe to the food we eat. Understanding basic chemistry concepts can help us appreciate the world around us and make informed decisions. In this article, we will explore five essential chemistry tips that everyone should know. Whether you're a student, a professional, or simply a curious individual, these tips will provide you with a deeper understanding of chemistry and its applications.
Chemistry is all around us, and it plays a crucial role in many industries, including healthcare, technology, and environmental conservation. By learning about chemistry, we can gain a better understanding of how things work and how we can improve our lives. From the medicines we take to the fuels we use, chemistry is an integral part of our daily routines. In the following sections, we will delve into five chemistry tips that will help you appreciate the importance of chemistry in our lives.
These tips will cover a range of topics, from the basics of chemical reactions to the applications of chemistry in everyday life. We will explore how chemistry can help us solve real-world problems, such as climate change and disease prevention. By the end of this article, you will have a better understanding of the power of chemistry and how it can be used to improve our world. So, let's get started and explore the fascinating world of chemistry.
Understanding Chemical Reactions
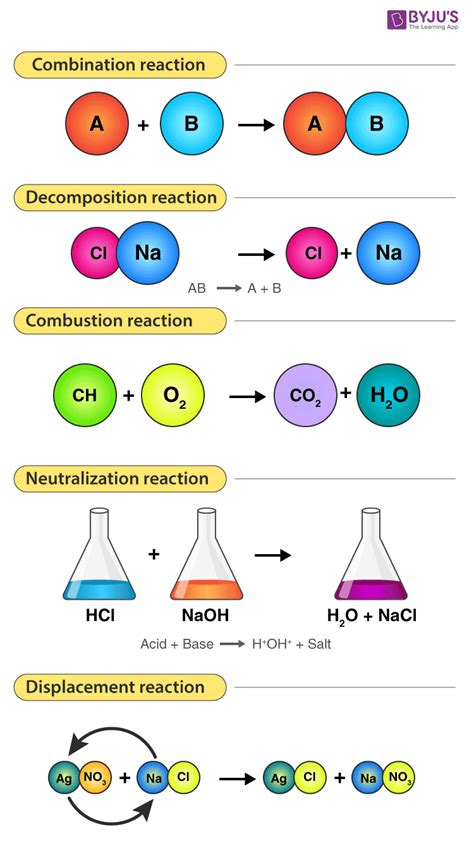
To understand chemical reactions, it's essential to know the basics of chemical equations. A chemical equation is a symbolic representation of a chemical reaction, using formulas and symbols to describe the reactants and products. Balancing chemical equations is a critical step in understanding chemical reactions, as it ensures that the number of atoms of each element is conserved during the reaction. By mastering chemical equations and reactions, you can gain a deeper understanding of the chemical processes that occur in our world.
Types of Chemical Reactions
There are several types of chemical reactions, each with its unique characteristics and applications. Synthesis reactions involve the combination of two or more substances to form a new compound. Decomposition reactions involve the breakdown of a single substance into two or more simpler substances. Replacement reactions involve the substitution of one element for another in a compound. Understanding the different types of chemical reactions can help you appreciate the diversity of chemical processes that occur in our world.Some examples of chemical reactions include:
- Combustion reactions, which involve the burning of fuels to produce heat and light
- Oxidation reactions, which involve the loss of electrons by a substance
- Reduction reactions, which involve the gain of electrons by a substance
- Acid-base reactions, which involve the transfer of protons between substances
Acids and Bases
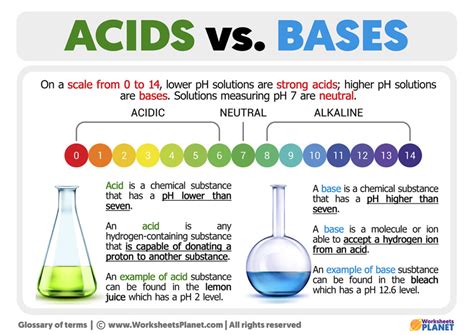
Acids and bases have many applications in our daily lives, from the food we eat to the medicines we take. For example, citric acid is a common ingredient in many food products, while baking soda is a base used in baking and cooking. Understanding the properties and behavior of acids and bases can help you appreciate the importance of chemistry in our daily lives.
pH Scale
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with pH 7 being neutral. The pH scale is logarithmic, meaning that each step up or down the scale represents a tenfold change in acidity or basicity. Understanding the pH scale is essential for many applications, from environmental monitoring to medical research.Some examples of acids and bases include:
- Hydrochloric acid, a strong acid found in stomach acid
- Sodium hydroxide, a strong base used in soap and detergent manufacturing
- Acetic acid, a weak acid found in vinegar
- Ammonia, a weak base used in cleaning products
Chemical Bonding
Understanding chemical bonding is essential for many applications, from materials science to pharmaceutical research. By mastering chemical bonding, you can gain a deeper understanding of the structure and properties of molecules.
Types of Chemical Bonds
There are several types of chemical bonds, each with its unique characteristics and applications. Covalent bonds are the most common type of chemical bond, involving the sharing of electrons between atoms. Ionic bonds involve the transfer of electrons between atoms, resulting in the formation of ions. Metallic bonds involve the delocalization of electrons in a metal lattice, resulting in high electrical conductivity.Some examples of chemical bonds include:
- Hydrogen bonds, which involve the attraction between hydrogen atoms and other atoms
- Van der Waals bonds, which involve the attraction between molecules
- Peptide bonds, which involve the linkage of amino acids in proteins
- Disulfide bonds, which involve the linkage of cysteine residues in proteins
Lab Safety

Lab safety is not only important for chemists but also for anyone who works with chemicals or hazardous materials. By mastering lab safety protocols, you can minimize the risk of accidents and ensure a safe working environment.
Lab Safety Equipment
Lab safety equipment is essential for preventing accidents and ensuring a safe working environment. Some common lab safety equipment includes gloves, goggles, lab coats, and fume hoods. Understanding the proper use of lab safety equipment is critical for minimizing the risk of accidents.Some examples of lab safety equipment include:
- Gloves, which protect hands from chemical splashes and spills
- Goggles, which protect eyes from chemical splashes and spills
- Lab coats, which protect clothing from chemical splashes and spills
- Fume hoods, which remove hazardous fumes and particles from the air
Environmental Chemistry

By mastering environmental chemistry, you can gain a deeper understanding of the chemical processes that occur in the environment and how human activities affect the environment. Some examples of environmental chemistry include:
- Climate change, which involves the increase in atmospheric carbon dioxide and other greenhouse gases
- Air pollution, which involves the release of pollutants into the atmosphere
- Water pollution, which involves the release of pollutants into waterways
- Soil pollution, which involves the release of pollutants into soil
Chemistry Image Gallery







What is chemistry?
+Chemistry is the study of the composition, properties, and reactions of matter.
Why is chemistry important?
+Chemistry is important because it helps us understand the world around us and develop new technologies and products.
What are some common chemistry applications?
+Some common chemistry applications include medicine, technology, environmental conservation, and energy production.
How can I learn more about chemistry?
+You can learn more about chemistry by taking online courses, reading books and articles, and participating in chemistry experiments and projects.
What are some common chemistry misconceptions?
+Some common chemistry misconceptions include the idea that chemistry is only about mixing chemicals and creating explosions, and that chemistry is not relevant to everyday life.
We hope you have enjoyed this article on 5 Chem Tips and have gained a deeper understanding of the importance of chemistry in our lives. Chemistry is a fascinating subject that has many applications and implications for our daily lives. By mastering chemistry, you can gain a better understanding of the world around you and develop new skills and knowledge. We encourage you to continue learning about chemistry and to share your knowledge with others. You can also comment below with any questions or topics you would like to discuss further. Thank you for reading, and we look forward to hearing from you!
