Intro
Discover the surprising truth about aluminums brittleness. Learn the 5 ways aluminum can be brittle, including thermal shock, grain boundary issues, and alloy composition. Understand the causes of aluminum brittleness and how it affects durability, corrosion resistance, and overall performance in various industrial applications.
Aluminum is a widely used metal in various industries due to its unique properties, such as its high strength-to-weight ratio, corrosion resistance, and conductivity. However, like any other metal, aluminum is not immune to brittleness, which can lead to unexpected failures and compromised structural integrity. In this article, we will explore five ways aluminum can be brittle and discuss the underlying causes, effects, and potential mitigation strategies.
1. Hydrogen Embrittlement
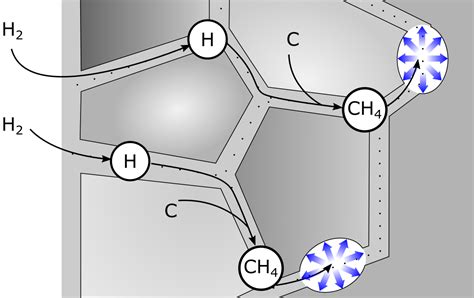
Hydrogen embrittlement is a common cause of brittleness in aluminum alloys, particularly those containing magnesium and silicon. When aluminum is exposed to hydrogen, it can absorb the gas, leading to the formation of hydrogen bubbles within the metal's crystal structure. As the bubbles grow, they can cause the aluminum to become brittle, leading to cracking and failure.
The primary source of hydrogen embrittlement in aluminum is the pickling process, where the metal is treated with acid to remove surface impurities. Other factors, such as welding, heat treatment, and exposure to moisture, can also contribute to hydrogen embrittlement.
Causes and Effects of Hydrogen Embrittlement
- Causes:
- Pickling process
- Welding and heat treatment
- Exposure to moisture
- Effects:
- Brittle cracking and failure
- Reduced ductility and toughness
- Compromised structural integrity
2. Corrosion Fatigue

Corrosion fatigue is another mechanism that can lead to brittleness in aluminum. When aluminum is exposed to corrosive environments, such as seawater or acidic solutions, it can undergo a process called pitting corrosion. Pitting corrosion creates small cracks and pits on the surface of the metal, which can act as stress concentrators and lead to brittle failure.
Causes and Effects of Corrosion Fatigue
- Causes:
- Exposure to corrosive environments
- Pitting corrosion
- Cyclic loading and stress
- Effects:
- Brittle cracking and failure
- Reduced fatigue life
- Compromised structural integrity
3. Over-Aging
Over-aging is a process that occurs when aluminum alloys are heat-treated for too long or at too high a temperature. This can cause the metal's microstructure to become unstable, leading to the formation of brittle precipitates and a reduction in ductility.
Over-aging can occur in various aluminum alloys, including 6061, 7075, and 2024. The effects of over-aging can be mitigated by controlling the heat treatment process and using techniques such as quenching and aging.
Causes and Effects of Over-Aging
- Causes:
- Overheating or prolonged heat treatment
- Unstable microstructure
- Effects:
- Reduced ductility and toughness
- Brittle cracking and failure
- Compromised structural integrity
4. Cold Working

Cold working is a process that involves deforming aluminum at room temperature, typically through rolling, bending, or stretching. While cold working can improve the metal's strength and hardness, it can also lead to brittleness.
Cold working can cause the metal's microstructure to become distorted, leading to the formation of dislocations and grain boundary cracks. These defects can act as stress concentrators and lead to brittle failure.
Causes and Effects of Cold Working
- Causes:
- Deformation at room temperature
- Distorted microstructure
- Dislocations and grain boundary cracks
- Effects:
- Reduced ductility and toughness
- Brittle cracking and failure
- Compromised structural integrity
5. Alloy Composition
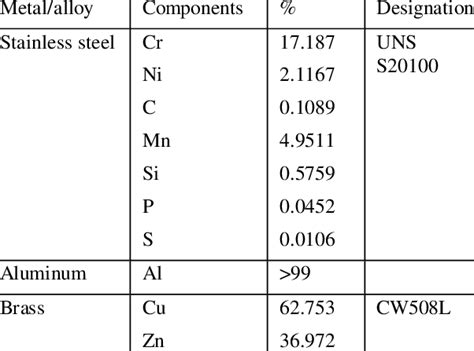
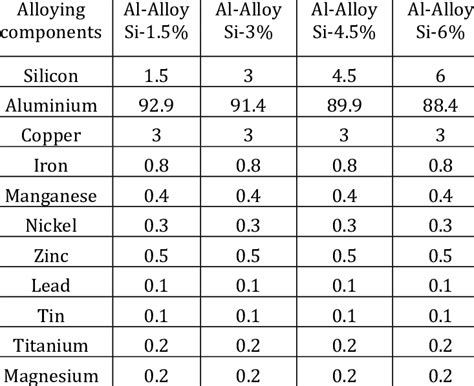
The composition of aluminum alloys can also affect their brittleness. Certain alloying elements, such as copper, magnesium, and silicon, can improve the metal's strength and corrosion resistance but may also contribute to brittleness.
For example, aluminum alloys containing high levels of copper, such as 2024 and 6061, can be prone to brittleness due to the formation of brittle precipitates. Similarly, alloys containing high levels of magnesium, such as 5083 and 5454, can be susceptible to corrosion fatigue and brittle failure.
Causes and Effects of Alloy Composition
- Causes:
- Alloying elements (e.g., copper, magnesium, silicon)
- Formation of brittle precipitates
- Effects:
- Reduced ductility and toughness
- Brittle cracking and failure
- Compromised structural integrity
Gallery of Aluminum Brittleness
Aluminum Brittleness Image Gallery





What is the primary cause of brittleness in aluminum alloys?
+The primary cause of brittleness in aluminum alloys is hydrogen embrittlement, which occurs when the metal absorbs hydrogen, leading to the formation of brittle precipitates and cracking.
How can over-aging affect the brittleness of aluminum alloys?
+Over-aging can cause the metal's microstructure to become unstable, leading to the formation of brittle precipitates and a reduction in ductility.
What is the effect of cold working on the brittleness of aluminum alloys?
+Cold working can cause the metal's microstructure to become distorted, leading to the formation of dislocations and grain boundary cracks, which can act as stress concentrators and lead to brittle failure.
In conclusion, brittleness in aluminum alloys can arise from various mechanisms, including hydrogen embrittlement, corrosion fatigue, over-aging, cold working, and alloy composition. Understanding the underlying causes and effects of these mechanisms is crucial for developing strategies to mitigate brittleness and ensure the structural integrity of aluminum components.
