Intro
Discover the 5 Ways Periodic Table simplifies chemistry with elemental organization, periodic trends, and chemical properties, making it a foundational tool for understanding atomic structure and chemical reactions.
The periodic table is a fundamental tool in chemistry, providing a comprehensive framework for understanding the properties and relationships of elements. Its significance extends beyond the realm of chemistry, influencing various fields such as physics, biology, and materials science. The periodic table's impact on our daily lives is profound, from the development of new technologies to the improvement of healthcare and environmental sustainability. As we delve into the world of elements, it becomes clear that the periodic table is an indispensable resource for scientists, researchers, and students alike.
The periodic table's importance stems from its ability to organize elements in a logical and systematic manner. By arranging elements in order of increasing atomic number, the periodic table reveals patterns and trends that help us understand the behavior of elements. This understanding is crucial for predicting the properties of elements, identifying potential applications, and developing new materials. Furthermore, the periodic table provides a common language for chemists and scientists, facilitating communication and collaboration across disciplines.
The periodic table's influence on modern society is multifaceted. From the development of semiconductors and electronics to the creation of new medicines and materials, the periodic table has played a pivotal role in shaping our technological advancements. Additionally, the periodic table has helped us better understand the natural world, from the composition of rocks and minerals to the behavior of atoms and molecules. As our understanding of the periodic table continues to evolve, we can expect to see new breakthroughs and innovations that transform our daily lives.
Introduction to the Periodic Table
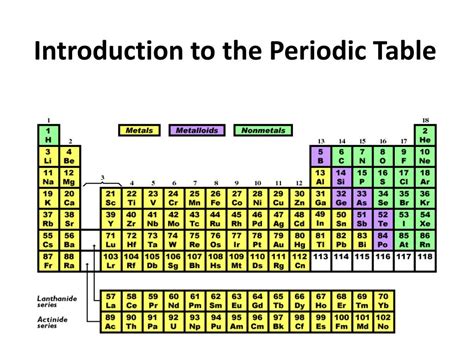
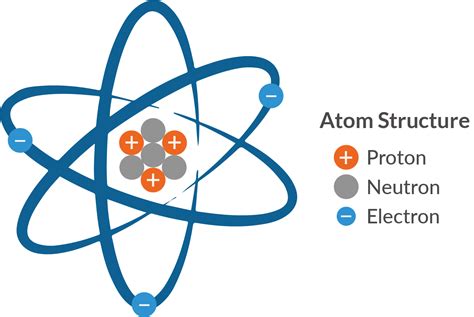
The periodic table is a tabular arrangement of elements, with rows called periods and columns called groups. Elements are arranged in order of increasing atomic number, which represents the number of protons in an atom's nucleus. The periodic table is divided into several blocks, including the s-block, p-block, d-block, and f-block, each corresponding to a specific type of orbital. Understanding the periodic table's structure and organization is essential for navigating its vast array of elements and predicting their properties.
History of the Periodic Table
The periodic table has a rich history, dating back to the early 19th century. The first periodic tables were developed by scientists such as John Newlands and Dmitri Mendeleev, who recognized patterns and relationships between elements. Over time, the periodic table has undergone significant revisions, with the addition of new elements and the refinement of existing ones. Today, the periodic table is a dynamic and evolving tool, with new discoveries and advancements continually expanding our understanding of the elements.Understanding the Periodic Table
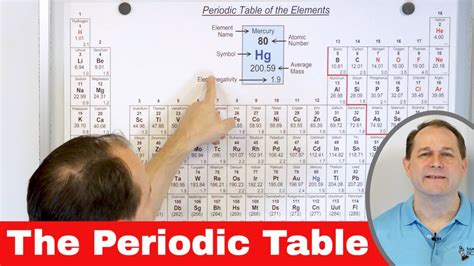
To fully appreciate the periodic table's significance, it is essential to understand its underlying principles and concepts. This includes familiarity with atomic structure, electron configuration, and chemical bonding. By grasping these fundamental concepts, individuals can better navigate the periodic table and unlock its secrets. Additionally, understanding the periodic table's limitations and challenges is crucial for appreciating its complexities and nuances.
Key Concepts and Principles
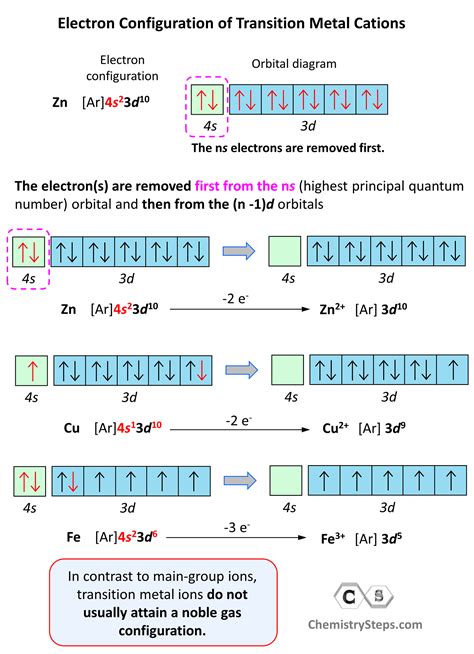
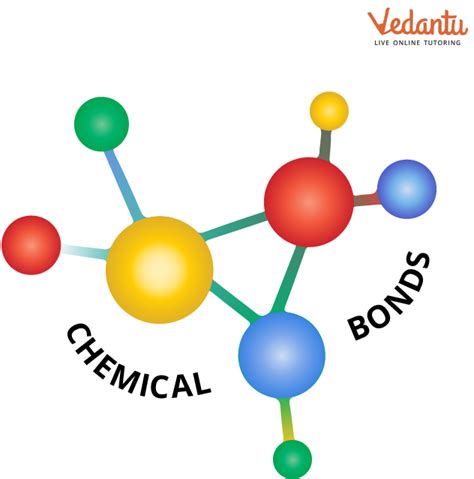
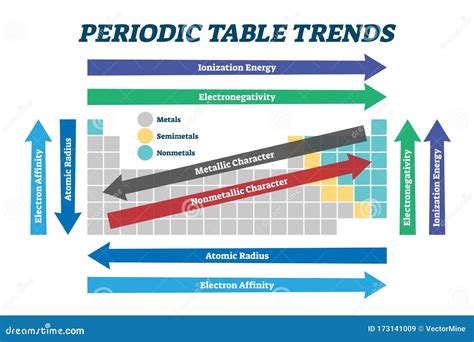
Some key concepts and principles underlying the periodic table include: * Atomic structure: The arrangement of protons, neutrons, and electrons within an atom. * Electron configuration: The distribution of electrons within an atom's orbitals. * Chemical bonding: The formation of bonds between atoms, resulting in the creation of molecules. * Periodic trends: Patterns and relationships between elements, such as electronegativity and atomic radius.Applications of the Periodic Table
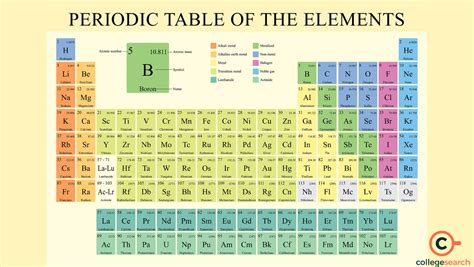
The periodic table has numerous applications across various fields, including:
- Materials science: The development of new materials with unique properties, such as superconductors and nanomaterials.
- Chemistry: The prediction of chemical reactions and the synthesis of new compounds.
- Physics: The understanding of atomic and subatomic phenomena, such as nuclear reactions and particle physics.
- Biology: The study of biomolecules and the behavior of elements in living organisms.
Real-World Examples
Some real-world examples of the periodic table's applications include: * The development of semiconductors and electronics, which rely on the properties of elements such as silicon and germanium. * The creation of new medicines, such as antibiotics and vaccines, which often involve the use of elements such as carbon, hydrogen, and oxygen. * The improvement of environmental sustainability, through the development of renewable energy sources and the reduction of pollution.Challenges and Limitations

Despite its significance, the periodic table is not without its challenges and limitations. Some of these include:
- The existence of unknown or undiscovered elements, which can limit our understanding of the periodic table's completeness.
- The complexity of certain elements, such as radioactive or highly reactive elements, which can make them difficult to study and understand.
- The need for ongoing research and refinement, as new discoveries and advancements continually expand our understanding of the elements.
Future Directions
As our understanding of the periodic table continues to evolve, we can expect to see new breakthroughs and innovations that transform our daily lives. Some potential future directions include: * The discovery of new elements, which can expand our understanding of the periodic table's completeness. * The development of new materials and technologies, which can leverage the unique properties of elements to create innovative solutions. * The improvement of environmental sustainability, through the development of renewable energy sources and the reduction of pollution.Conclusion and Final Thoughts

In conclusion, the periodic table is a powerful tool that has revolutionized our understanding of the elements and their properties. Its significance extends beyond the realm of chemistry, influencing various fields and transforming our daily lives. As we continue to explore and refine the periodic table, we can expect to see new breakthroughs and innovations that shape the future of science and technology.
Periodic Table Image Gallery
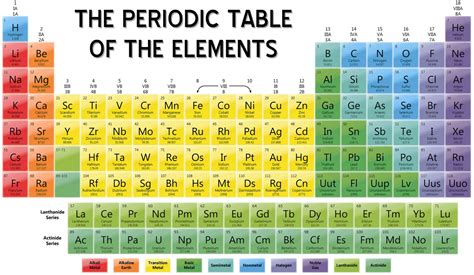




What is the periodic table?
+The periodic table is a tabular arrangement of elements, with rows called periods and columns called groups.
What are the applications of the periodic table?
+The periodic table has numerous applications across various fields, including materials science, chemistry, physics, and biology.
What are the challenges and limitations of the periodic table?
+The periodic table is not without its challenges and limitations, including the existence of unknown or undiscovered elements and the complexity of certain elements.
How does the periodic table impact our daily lives?
+The periodic table has a profound impact on our daily lives, from the development of new technologies to the improvement of healthcare and environmental sustainability.
What is the future of the periodic table?
+The future of the periodic table is exciting, with new breakthroughs and innovations expected to transform our understanding of the elements and their properties.
We hope this article has provided you with a comprehensive understanding of the periodic table and its significance. Whether you are a student, researcher, or simply interested in science, the periodic table is an indispensable resource that can help you navigate the complex world of elements. We encourage you to share your thoughts and questions in the comments section below, and to explore the many resources available for further learning and discovery.

